
-
Zeolite HC-SCR catalyst2026-01
The Zeolite HC-SCR catalyst is a catalytic material that utilizes hydrocarbons (HC) as a reducing agent to selectively reduce nitrogen oxides (NOx) to N₂ under lean (oxygen-rich) conditions. Manufactured using a Zeolite as the support material loaded with metal active components, this catalyst's core function is to directly leverage unburned hydrocarbons present in exhaust gas as the reducing agent for NOx conversion to N₂.
Chemical Reactions:
1、Main SCR Reaction:
CH₄ + 2NO + O₂ → N₂ + CO₂ + 2H₂O2、HC Oxidation Side Reaction:
CH₄ + 2O₂ → CO₂ + 2H₂OThis catalyst not only simplifies system architecture but also simultaneously treats both NOx and HC emissions, achieving a "dual-function, single-unit" solution.
While conventional zeolite-based HC-SCR catalysts theoretically offer system simplification advantages, efficiency and stability limitations have restricted their application to experimental use in passenger vehicles, with urea-SCR technology remaining the mainstream solution.
However, TES has recently developed a novel HC-SCR catalyst that employs a new, stable Zeolite structure as the support, impregnated with a composite metallic material as the active component. This innovation provides the catalyst with excellent selectivity for HC-to-NOx conversion under lean conditions, along with enhanced sulfur resistance.
Test Gas Conditions:
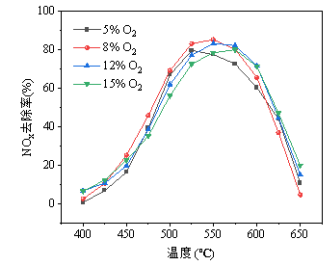
Figure 1 (Oxygen concentration effect): Under low-oxygen conditions (5–8% O₂), the catalyst achieves 80–100% NOx conversion in the 450–650°C temperature range; however, high-oxygen environments (12–15% O₂) significantly suppress catalytic activity, particularly in the medium-temperature zone of 400–500°C, where the conversion rate drops by 20–40 percentage points, indicating that this catalyst is more suitable for oxygen-deficient or fuel-rich operating conditions.
Test Gas Conditions:
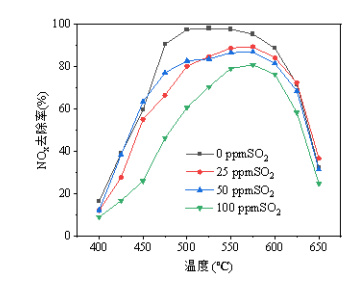
Figure 2 (SO₂ effect): SO₂ exerts a slight inhibitory effect on the catalyst; 100 ppm SO₂ reduces the conversion rate by approximately 5–15 percentage points, but this impact gradually diminishes as the temperature rises above 600°C; the effect of low-concentration SO₂ (25–50 ppm) is relatively minor, suggesting the catalyst possesses certain sulfur resistance, though the risk of sulfur poisoning should be considered during long-term operation.
The gas conditions for the test are:
NO content at 500 ppm, CH4 content at 800 ppm, O2 concentration at 8%, space velocity at 40,000 h^-1, reaction temperature range of 400-650 ℃, and SO2 content set at 0 ppm, 25 ppm, 50 ppm, and 100 ppm.
This breakthrough system utilizes hydrocarbons present in exhaust gas to reduce NOx emissions, offering a cost-effective solution, especially, for the baking industry's after-treatment requirements.